TRADITIONAL REMEDY FACING NEW CHALLANGES
Turmeric (Curcuma longa L.) has been used in India for more than 5000 years as a spice and medicinal herb in traditional ayurvedic medicine. Well-known for its anti-inflammatory properties, it is now recognized as the #1 botanical for healthy ageing. However, its poor bioavailability (amount of actives reaching the blood stream) in curcuminoids lowers its efficacy and its poor solubility limits its application (often limited to capsules and tablets), therefore high intake (~1500mg) of standard turmeric extract was shown to be efficient.
THE FOOD MATRIX AND CLEAN LABEL TREND
iosolve Optimized Curcumin has overcome all these issues with a unique formulation that preserve Turmeric original natural composition, it offers enhanced bioavailability and efficacy at only 500mg/day without compromising the purity and the quality of its Food Matrix.
Zeus Hygia is able to encapsulate water insoluble actives in food grade water soluble polysaccharide matrix (modified starch) following a spray drying process.
PRODUCT APPLICATIONS
The food matrix of this ingredient makes it perfect for any food and food supplement application. Enhanced solubility allows the inclusion into Powders, Bars, Hot/Cold Beverages, Ice Creams, dairy.
INSTANT BEVERAGES
SHOTS
GUMMIES
CAPSULES
& MANY MORE
BIOAVAILABILITY
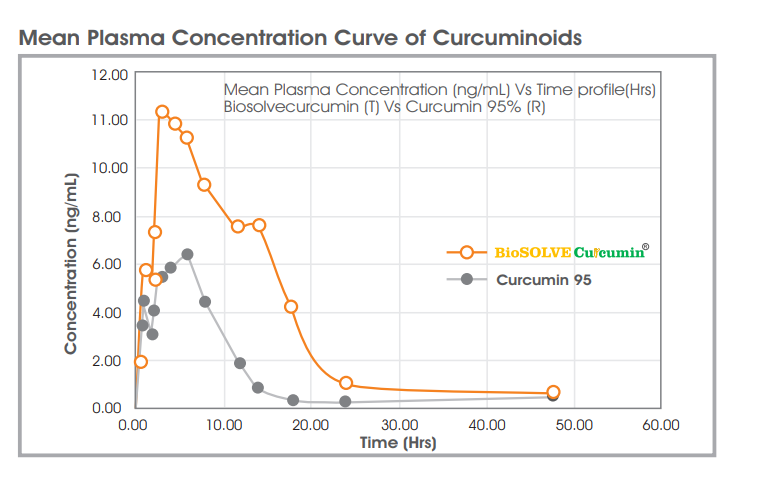
Biosolve is 8X (AUC) times more bioavailable than standard curcumin 95% while avoiding all nasty synthetic ingredients, emulsifying agents, surfactants, and gastric irritants like black pepper.
This Hydrophilic carrier-based encapsulation system possesses unique properties which in-turn releases the bioactive molecules as uniform, finer particles with larger surface area in the GI system. Increased aqueous solubility with better dissolution properties in the intestinal fluids supports enhanced absorption of Biosolve curcumin from the Gut wall.
30 days
- 25% reduction in pain (VAS)
- WOMAC Pain: statistically significant within and between group p values (p=0.0001) after 30 days trend, and continued to 90 days
30 days
- 42% reduction in pain (VAS)
- ACR 20-physical function Index (FN) improved by 20%. Womac index (pain, stiffness, physical function) shows significant improvement of 23%.
30 days
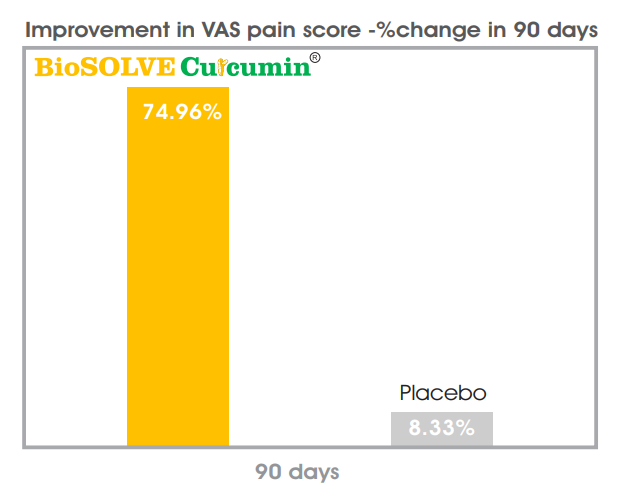
- 75% reduction in pain (VAS)
- ACR 20-physical function Index (FN) improved by 36%. Womac index (pain, stiffness, physical function) shows significant improvement of 57%.
CLINICAL STUDIES
Increasing health benefits over a period of 30, 60 and 90 days Clinical and pre-clinical studies show reduction in pain and stiffness while improving mobility and physical functions, while no adverse reactions